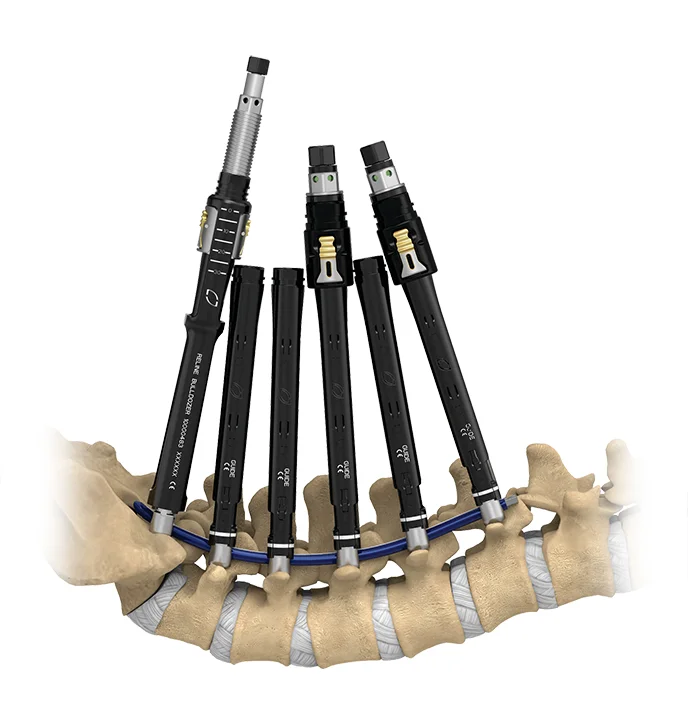
XLIF™
eXtreme Lateral Interbody Fusion
For over 20 years XLIF™ has helped to shape minimally invasive surgery through clinically validated, reproducible procedural workflows and continued innovation across technologies including access, intraoperative neuromonitoring, implant designs optimized for patient anatomy and fusion, and enabling technologies.
Advancing XLIF™. Expanding Capability.
Globus Medical is proud to advance XLIF™ into a new era of expanded capability across technique and approach. We offer one of the most comprehensive interbody portfolios in lateral surgery and our continued investment in procedurally integrating enabling technologies to improve workflow reproducibility and patient care.
Clinically Validated Technique
XLIF™ is designed to be effective at addressing pathologies at L4-L5 and above from the lateral decubitus position. Clinically validated, with over 500 peer-reviewed articles,1 XLIF™ has demonstrated more predictable outcomes than traditional posterior spinal fusion procedures, with substantially fewer complications.2-6
Reduction in operative time7
Fusion rate8
Achievement of indirect decompression9
Reduction in infection rates10
Reduction in blood loss2, 11-13
Reduction in revision rates10
Reduction in hospital stay10
Comprehensive Procedural Solution
Globus Medical provides a comprehensive procedural portfolio including XLIF™, XLIF™ Thoracic, XLIF™ ACR™, XLIF™ Corpectomy, XLIF™ Prone, and XLIF™ Lateral SPS to address numerous pathologies with one surgical procedure.
Access and Positioning
Integrated Access, Monitoring, and Positioning
Control, stability, and simplicity integrate with intraoperative neuromonitoring to provide customizable access and actionable information throughout the XLIF™ procedure.

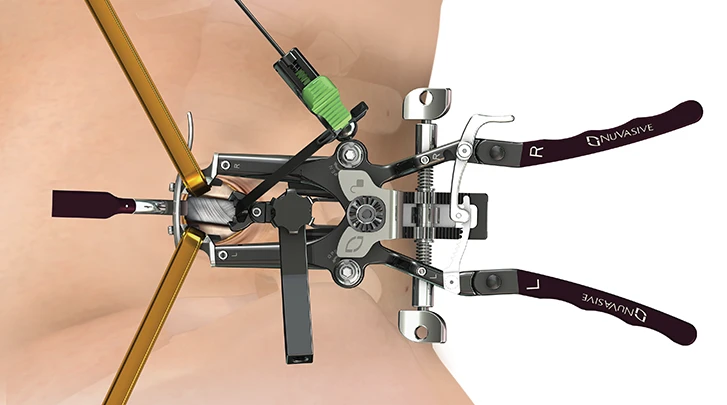
MaXcess™ 4
The MaXcess™ 4 access system combines incremental retraction, stability, ergonomics, and strength to enable optimized access to relevant anatomy, while decreasing unnecessary tissue retraction. Integrated with NVM5™ neuromonitoring, MaXcess™ 4 provides real-time discrete thresholds, directionality, and relative proximity to guide surgeons throughout the procedure from traversing the psoas through closure, providing access at L4-L5 and above.

MARS™3VL
The simplified design and enhanced functionality of MARS™3VL allows the freedom to focus more on the surgery and less on the setup. The retractor is engineered for efficient and controlled access to the disc space and is tailored to accommodate various surgeon preferences with radiolucent blades securely attached to the stainless steel frame.
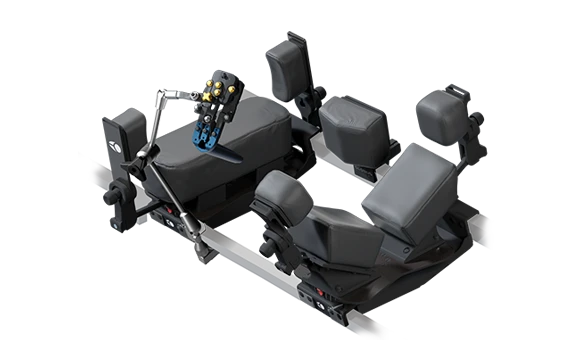
Prone Lateral Patient Positioning System
The Prone Lateral Patient Positioning System is a comprehensive solution allowing for adaptable surgical access, reliable stability, and surgeon ergonomics. The positioner is an important component of the Excelsius™ Prone Lateral Surgical Solution, with multiple features to facilitate prone lateral procedures with ease.
Interbody Implants
Comprehensive Lateral Interbody Spacer Portfolio
We deliver versatility and the ability to achieve surgical goals through one of the most comprehensive portfolios of expandable and Advanced Materials Science™ interbody spacer technologies.
Advanced Materials Science™
Adhering to the three core principles of Advanced Materials Science™—surface, structure, and imaging—we have pioneered design and manufacturing methods that combine the inherent benefits of porosity with the advantageous material properties of PEEK and titanium.
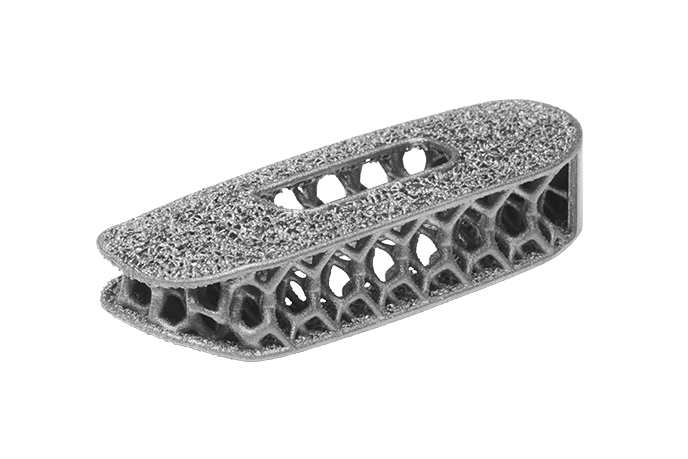
Modulus™ XLIF™
Modulus™ XLIF™ integrates endplate porosity with an optimized body lattice structure designed to provide a fully porous architecture and favorable environment for bone ingrowth.14 The architecture results in an effective modulus similar to bone and reduces material to provide optimized imaging clarity.

Cohere™ XLIF™
Cohere™ XLIF™ is a lateral porous PEEK interbody spacer designed to promote bone ingrowth15 while maintaining the biomechanical15 and imaging properties of PEEK.

HEDRON L™
HEDRON L™ is a 3D-printed titanium interbody spacer that features a biomimetic, porous scaffolding designed to promote bone formation onto and through the implant.*
*Animal study data on file.
Expandable Technology
Our comprehensive lateral interbody expandable solutions are designed to minimize disruption to patient anatomy and maximize segmental lordosis while also helping to restore and preserve sagittal alignment.
Corpectomy
Expandable and static vertebral body replacement solutions enable customization for complex cases that require anterior column support and restoration of sagittal balance.
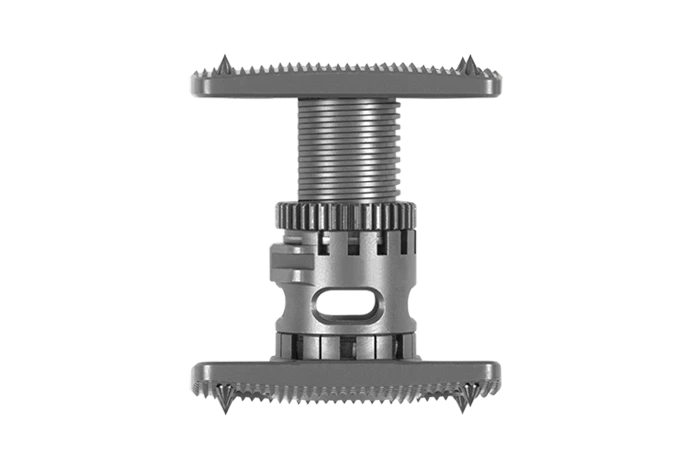
FORTIFY™
FORTIFY™ streamlines vertebral body replacement through one of several approaches and provides a range of implants designed to restore height, alignment, and stability. PEEK or titanium materials, maximized expansion ranges, and modular endplates allow surgeons to customize each implant for their patient.

XCore™ 2
The XCore™ 2 vertebral body replacement is designed to be a versatile and advanced expandable corpectomy vertebral body replacement that will allow surgeons to utilize novel XLIF™ end caps from multiple lateral approaches.
Fixation
Powerful Fixation Options
Versatility meets efficiency through our suite of powerful lateral plating and posterior fixation systems.
Plates
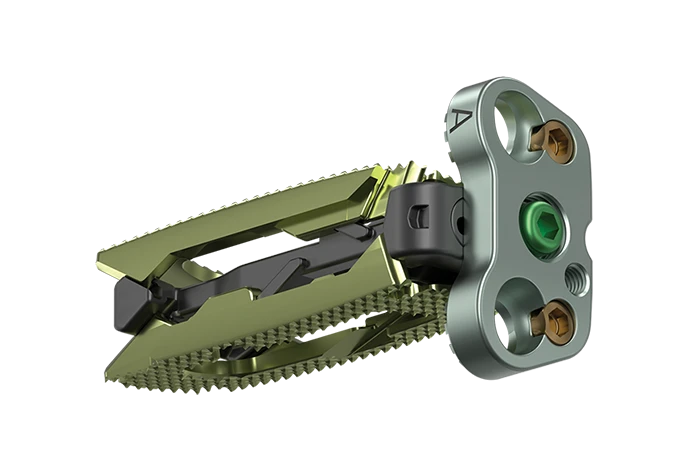
ADIRA™ XLIF™ Lateral Plate
The ADIRA™ XLIF™ Lateral Plate System is designed to refine lateral plating by offering simplified insertion workflows and a rigid coupling mechanism to confidently align plates over RISE-L™, Modulus™ XLIF™, Hedron L™, Cohere™ XLIF™, TransContinental™, and CoRoent™ XLIF™ interbody spacers, to enhance construct stability.

Modulus™ XLIF™ Plate
The Modulus™ XLIF™ Plate is a low-profile anti-migration device designed for use with the Modulus™ XLIF™ interbody spacer to provide stability in multi-stage procedures or in the event of an anterior longitudinal ligament rupture.

Cohere™ XLIF™ AMS Plate
The Cohere™ XLIF™ AMS Plate is a low-profile anti-migration device designed for use with the Cohere™ XLIF™ interbody spacer to provide stability in multi-stage procedures or in the event of an anterior longitudinal ligament rupture.
Posterior Fixation
CREO™
CREO™ offers a complete array of thoracolumbar stabilization implants for treating a range of spinal pathologies. Designed to enhance procedural efficiency and ease of use, this system features a threaded locking mechanism, low-profile implants, and intuitive instruments to accommodate multiple rod diameters.
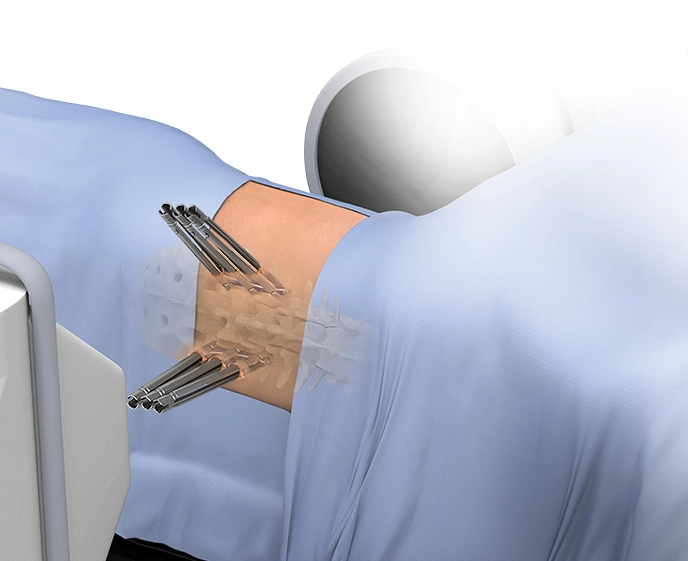
XFixation™
XFixation™ is an adapted posterior fixation procedure with the patient kept in a lateral decubitus position. This allows single-position surgery to be achieved from the upper thoracic spine to the sacrum when combined with XLIF™ and XALIF™. Robotically navigated lateral posterior fixation with ExcelsiusGPS™ assists with challenging trajectories and provides improved ergonomics from the lateral approach.
Enabling Technologies
Industry-Leading Neuromonitoring, Navigation, Robotics, and Power Portfolio
Our comprehensive lateral offering is procedurally integrated with industry-leading enabling technology and power platforms to deliver intelligence, reproducible workflows, efficiency, and confidence to meet surgeon and patient needs.
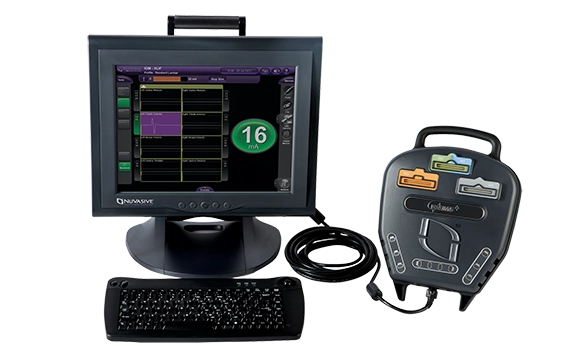
NVM5™
Neuromonitoring with NVM5™ is a foundational requirement in every XLIF™ case. Our services and technology provide proprietary automated nerve detection with standardized setup and clinically validated alerts16 to help reduce variability and allow for fast interpretation of neural information.

Power Portfolio
The Globus Medical Power Portfolio is engineered to seamlessly integrate with enabling technology to streamline surgical workflow and address clinical challenges. The portfolio drives efficiency at every level and helps to safeguard critical and delicate anatomy through navigation for enhanced visualization and through innovative technologies like the DuraPro™ Oscillating System, designed to remove bone without wrapping tissue.
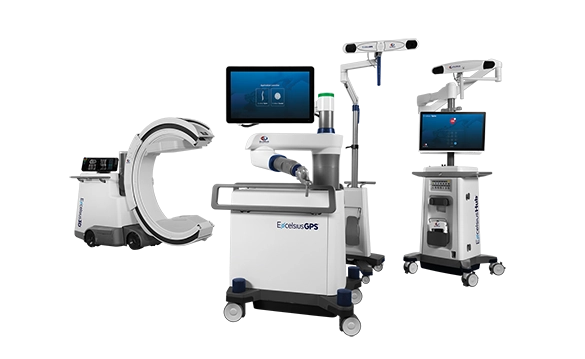
Excelsius™ Technology
Excelsius™ Technology is an ecosystem of technologies designed to improve safety and care in the OR. The uniquely intelligent features that define ExcelsiusGPS™, Excelsius3D™, and ExcelsiusHub™ are procedurally integrated to enhance safety and accuracy.
Regenerative Biologics
Comprehensive Regenerative Biologic Solutions*
Globus Medical is focused on pioneering innovative regenerative solutions for patients with musculoskeletal disorders. Our comprehensive biologics portfolio includes cellular allografts, synthetic bone void fillers, demineralized bone products, amniotic barriers, graft delivery systems, and autologous biologic centrifuge technologies.
*Indications/applications for use vary by product. Refer to the IFU for comprehensive product information.
Prioritizing Clinical Education
Over the past 20 years, we have evolved our programs in partnership with our renowned faculty. We provide learning experiences spanning online educational resources to surgeon-led didactic courses with hands-on training, mentorship opportunities, and field-based training support.
- Data on file.
- Lehmen JA, Gerber EJ. MIS lateral spine surgery: a systematic literature review of complications, outcomes, and economics. Eur Spine J. 2015;24(Suppl 3):287-313.
- Cheng I, Briseno MR, Arrigo RT, et al. Outcomes of two different techniques using the lateral approach for lumbar interbody arthrodesis. Global Spine J. 2015;5(4):308-314.
- Khajavi K, Shen A, Lagina M, et al. Comparison of clinical outcomes following minimally invasive lateral interbody fusion stratified by preoperative diagnosis. Eur Spine J. 2015;24(Suppl 3):322-330.
- Okuda S, Miyauchi A, Oda T, et al. Surgical complications of posterior lumbar interbody fusion with total facetectomy in 251 patients. J Neurosurg Spine. 2006;4(4):304-309.
- Scaduto AA, Gamradt SC, Yu WD, et al. Perioperative complications of threaded cylindrical lumbar interbody fusion devices: anterior versus posterior approach. J Spinal Disord Tech. 2003;16(6):502-507.
- Sembrano JN, Tohmeh A, Isaacs R, et al. Two-year comparative outcomes of MIS lateral and MIS transforaminal interbody fusion in the treatment of degenerative spondylolisthesis: part I: clinical findings. Spine. 2016;41(Suppl 8):S123-S132.
- Rodgers WB, Gerber EJ, Patterson JR. Fusion after minimally disruptive anterior lumbar interbody fusion: analysis of extreme lateral interbody fusion by computed tomography. SAS J. 2010;4:63-66.
- Gabel BC, Hoshide R, Taylor W. An algorithm to predict success of indirect decompression using the extreme lateral lumbar interbody fusion procedure. Cureus. 2015;7(9):e317.
- Lucio JC, VanConia RB, DeLuzio KJ, et al. Economics of less invasive spinal surgery: an analysis of hospital cost differences between open and minimally invasive instrumented spinal fusion procedures during the perioperative period. Risk Manag Healthc Policy. 2012;5:65-74.
- Dakwar E, Cardona RF, Smith DA, et al. Early outcomes and safety of the minimally invasive, lateral retroperitoneal transpsoas approach for adult degenerative scoliosis. Neurosurg Focus. 2010;28(3):E8.
- Dhall S, Wang MY, Mummaneni PV. Clinical and radiographic comparison of mini-open transforaminal lumbar interbody fusion with open transforaminal lumbar interbody fusion in 42 patients with long-term follow-up. J Neurosurg Spine. 2008;9(6):560-565.
- Whitecloud TS, Roesch WW, Ricciardi JE. Transforaminal interbody fusion versus anterior-posterior interbody fusion of the lumbar spine: a financial analysis. J Spinal Disord. 2001;14(2):100-103.
- Preclinical data on file. Data may not be representative of clinical results. TR 9604787.
- Torstrick FB, Safranski DL, Burkus JK, et al. Getting PEEK to stick to bone: the development of porous PEEK for interbody fusion devices. Tech Orthop. 2017;32:158-166.
- Tohmeh AG, Rodgers WB, Peterson MD. Dynamically evoked, discrete-threshold electromyography in the extreme lateral interbody fusion approach. J Neurosurg Spine. 2011;14(1):31-37.