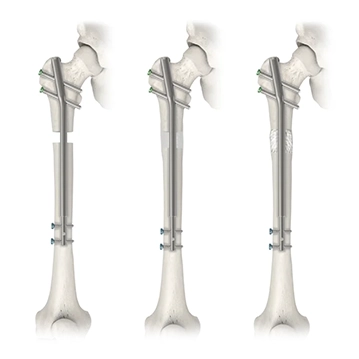
Limb Lengthening and Reconstruction
The Precice™ Intramedullary Limb Lengthening (IMLL) System is designed for limb lengthening, mal-unions, non-unions, or bone transport procedures. The innovative technology operates through a magnetic coupling with the implant and the handheld External Remote Controller (ERC) that can allow the nail to compress or distract depending on its application.
We aspire to enable surgeons to improve patients’ lives by making complex orthopedic reconstruction easier.
Precice™ is a proprietary technology that is adjustable from outside the body. This allows for constant changes at the time of implantation and over the course of treatment to accommodate the clinical needs of patients as they heal and grow. The technology is designed to give healthcare providers the ability to customize postoperative therapy for patients in a non-invasive manner using the External Remote Controller (ERC), reducing the need for further repeat surgical procedures, and promoting meaningful improvements in patient quality of life.

Clinical Benefits
The Precice™ Difference
Ease of Surgical Application
The same technique used to implant a standard trauma rod is used to implant Precice™1,2

Cost Savings
There was less total cost for limb length discrepancy patients treated with Precice™ compared to the previous standard of care3
Intraoperative Patient Customization
The surgeon can customize the implant with the fast distractor and use locking pegs and partially threaded or fully threaded screws1,2
Postoperative Patient Customization
The surgeon can customize the prescription for their patients with an External Remote Controller1,2

Standard of Care
More than 15,000 patients have been treated with Precice™ since 2011
How It Works
Technology Benefits
The Precice™ system provides a customized, less invasive* approach to limb lengthening with benefits that include:
- Proprietary magnet technology
- Precision rate control
- Non-invasive postoperative adjustment through the ERC
- Customizable lengthening protocol based upon the patient’s prescription
- Up to 80mm of non-invasive distraction capability
- Available in three diameters (8.5, 10.7, and 12.5mm)
- Device is reversible
*As compared to treatment with an external fixator
Learn More
State-of-the-Art Reconstruction Solutions
Precice™ Limb Lengthening System
The Precice™ IMLL System is an adjustable, state-of-the-art device that utilizes a remote control to non-invasively lengthen the femur or tibia with an ERC postoperatively. Interaction between magnets in the device and the ERC allows for precise, adjustable, and customizable distraction throughout the lengthening phase of treatment. Following the corticotomy and during the lengthening phase, the Precice™ implant is gradually lengthened externally with the handheld ERC based on patient requirements.
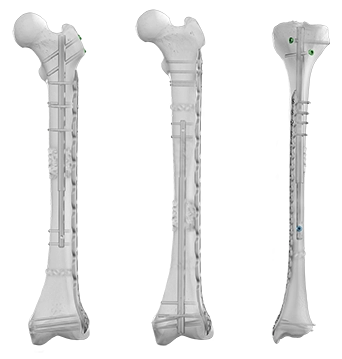
Plate Assisted Bone Segment Transport
Traditional bone transport treatment can be burdensome. Plate Assisted Bone Segment Transport (PABST) is a bone transport technique that combines the Precice™ nail with a locking plate to treat long bone defects, offering a simple, internal solution to treat segmental bone defects caused by a tumor or trauma.
Prioritizing Clinical Education
Over the past 20 years, we have evolved our programs in partnership with our renowned faculty. We provide learning experiences spanning online educational resources to surgeon-led didactic courses with hands-on training, mentorship opportunities, and field-based training support.
- Laubscher M, Mitchell C, Timms A, et al. Outcomes following femoral lengthening. An initial comparison of the Precice intramedullary lengthening nail and the LRS external fixator monorail system. Bone Joint J. 2016;98-B:1382-1388.
- Landge V, Shabtai L, Gesheff M, et al. Patient satisfaction after limb lengthening with internal and external devices. J Surg Orthop Adv. 2015;24(3):174-179.
- Richardson S, Schairer W, Fragomen A, et al. Cost comparison of femoral distraction osteogenesis with external lengthening over a nail versus internal magnetic lengthening nail. J Am Acad Orthop Surg. 2019;27(9):e430-e436.