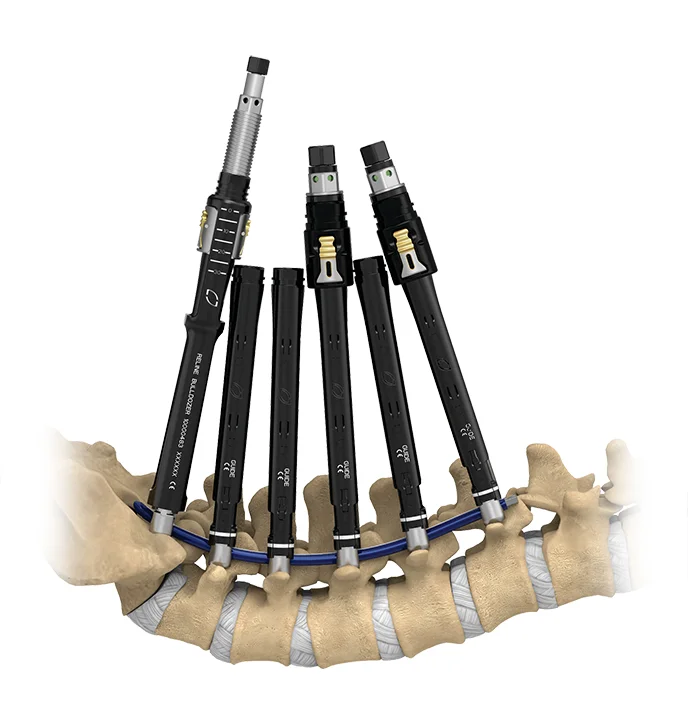
ALIF
Anterior Lumbar Interbody Fusion
The Globus ALIF procedural portfolio represents our commitment to advancing the ALIF procedure. Through optimized access, industry-leading interbody spacers, and versatile fixation paired with enabling technology, we provide access and spine surgeons with a comprehensive suite of integrated procedural solutions to accommodate surgeon preference and varying patient anatomy.
Advancing ALIF. Expanding Capability.
Comprehensive Procedural Solution
Globus Medical provides a comprehensive procedural portfolio including Supine ALIF, Lateral ALIF, and XLIF™ Lateral Single-Position Surgery to address numerous pathologies from anterior and lateral patient positioning.
Access
Rigid and Efficient Access
Access systems designed in collaboration with access surgeons combine strength, precision, visibility and a variety of instrument options to make ALIF access workflows more reproducible.
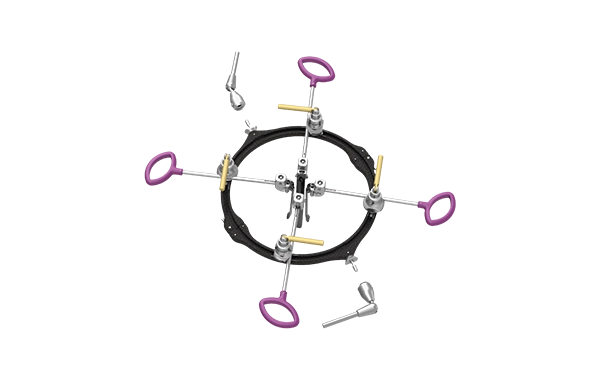
Supine ALIF
The Supine ALIF Retractor System offers an innovative retractor solution for ALIF access. The system features a dual-arm, table-mounted, carbon fiber frame and full suite of aluminum blades. This design provides both rigidity and radiolucency during ALIF procedures.
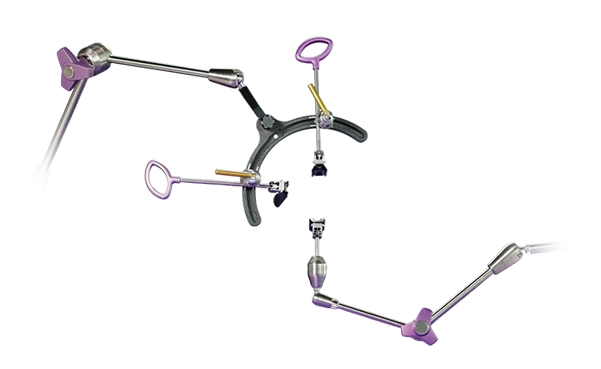
XALIF™
The XALIF™ Retractor System utilizes a similar surgical approach to traditional supine ALIF but is deployed with the patient in the lateral decubitus position. The versatile retractor system enables access to lower lumbar levels and reduces the need for patient repositioning. Eliminating patient repositioning has demonstrated a reduction in operating room times,1 patient time under anesthesia,2-3 and hospital costs.4-5
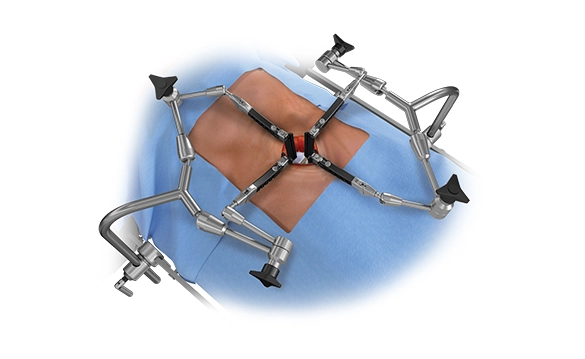
ALLEGIANCE™ Retractor System
The ALLEGIANCE™ Retractor System is a ringless anterior exposure system designed to provide rigid and reliable access for anterior spine procedures. The system features simplified instruments designed to obtain anterior access and reduce procedural steps. Individual table arms with a variety of blade offerings enable users to customize blade placement to help minimize the incision size while maintaining the desired exposure window.
Interbody Implants
Best-in-Class Interbody Spacer Portfolio
Unique, streamlined instruments and the best-in-class portfolio of fixated and non-fixated interbody implants including Advanced Materials Science™ and expandable technologies are designed to enhance surgical workflows and provide surgeons with operative flexibility.
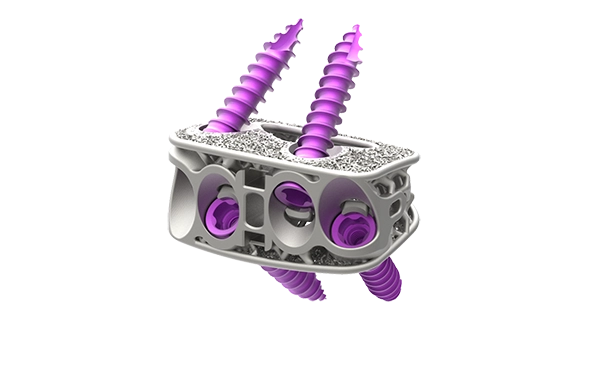
Modulus™ ALIF
Modulus™ ALIF is a porous titanium interbody spacer designed for both supine and lateral ALIF. Compatible with anchors and screws, the system provides implants in a variety of sizes and lordotic options to accommodate varying patient anatomy.

HEDRON IA™
HEDRON IA™ is compatible with anchors and screws, and features a biomimetic porous scaffolding designed to encourage cellular response and promote bone formation onto and through the implant.

Cohere™ ALIF
Cohere™ ALIF is a porous PEEK integrated anterior lumbar interbody fusion (ALIF) spacer intelligently designed to promote bone ingrowth while maintaining the biomechanical and imaging properties of PEEK.6,7

MONUMENT™
MONUMENT™ is a unique ALIF system with a built-in mechanical reduction feature designed to aid in spondylolisthesis reduction (up to Grade 2). The self-locking reduction feature simplifies the procedure as there is no additional step to lock the implant after the desired reduction has been achieved.

MAGNIFY™-S
MAGNIFY™-S is an all-titanium expandable ALIF spacer designed to maximize indirect decompression, restore disc height, and optimize fusion potential. Minimized insertion height and continuous expansion combined with the ability to pack additional bone graft after expansion makes MAGNIFY™-S an excellent choice for stand-alone* ALIF.
* Intended for stand-alone use in patients with DDD at one or two levels only when used with three screws per implant.

INDEPENDENCE MIS™
INDEPENDENCE MIS™ is an integrated lumbar interbody spacer designed to deliver screw or anchor fixation in fewer procedural steps through a less invasive surgical corridor than traditional integrated spacers.

CORBEL™ Lateral ALIF
CORBEL™ is an integrated plate-spacer interbody with offset MIS fixation to facilitate access to L5-S1 with the patient in the lateral position, eliminating the need to reposition the patient during the procedure.

HEDRON A™
HEDRON A™ is a 3D-printed titanium ALIF spacer that provides a porous environment within the spacer, which is unachievable with traditional manufacturing methods. The biomimetic porous scaffolding is designed to promote bone formation onto and through the implant.

CONTINENTAL™
The CONTINENTAL™ Spacer is an anterior lumbar interbody spacer made from PEEK radiolucent polymer, while CONTINENTAL™ TPS combines radiolucency with a titanium plasma spray (TPS) coated surface. The oval footprint and biconvex surfaces are designed to fit the vertebral endplates and maximize contact area.
Fixation
Versatile Fixation Options
Our portfolio of anterior plating and posterior fixation systems is designed for strength and versatility to accommodate surgeon preference and patient pathology.
Plates

VICTORY™ Plate
The VICTORY™ Lumbar Plating System offers low-profile implants and variability in screw angulation, which are designed to facilitate the insertion process. The system has 4-hole, 3-hole, and buttress plate options to accommodate various patient anatomies, procedural access windows, and surgeon preference.

Brigade™ ALIF Plate
The Brigade™ Plate leverages a single-step locking mechanism, variable screw trajectory, and multiple plate options to accommodate varying patient anatomies and surgeon preferences.
Posterior Fixation
CREO™
CREO™ offers a complete array of thoracolumbar stabilization implants for treating a range of spinal pathologies. Designed to enhance procedural efficiency and ease of use, this system features a threaded locking mechanism, low-profile implants, and intuitive instruments to accommodate multiple rod diameters.
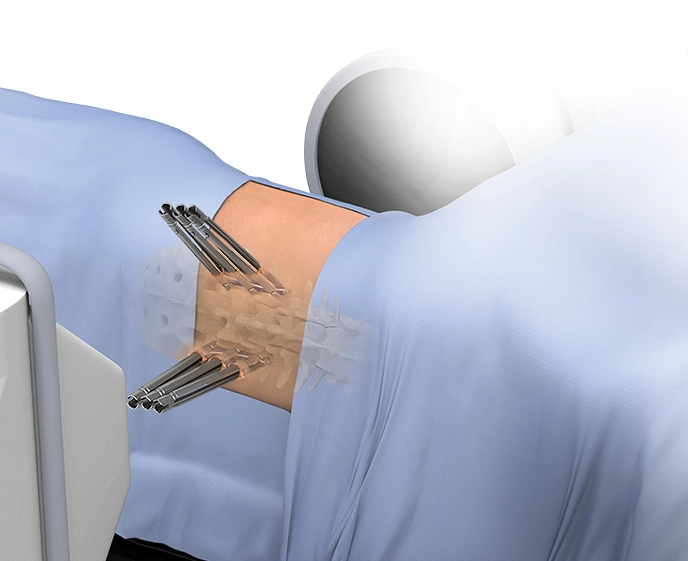
XFixation
XFixation is an adapted posterior fixation procedure with the patient kept in a lateral decubitus position. This allows single-position lateral surgery to be achieved from the upper thoracic spine to the sacrum when combined with XLIF™ and XALIF™. Robotically navigated lateral posterior fixation with ExcelsiusGPS™ assists with challenging trajectories and provides improved ergonomics from the lateral approach.
Enabling Technologies
Integrated Navigation, Robotics, and Power Portfolio
Rigid and efficient access, best-in-class interbody implants*, and versatile fixation integrate with industry-leading enabling technology and power platforms to deliver intelligence, reproducible surgical workflows, efficiency, and confidence.
* ExcelsiusGPS™ is not indicated for the placement of ALIF Interbody Spacers
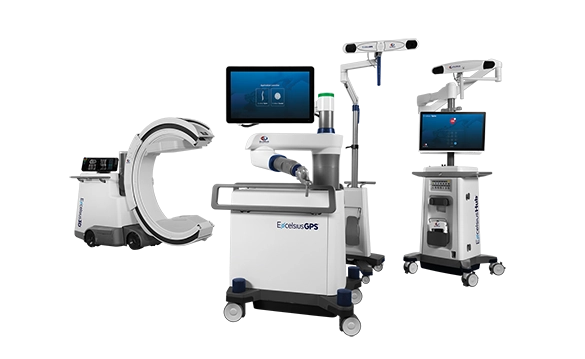
Excelsius™ Technology
Excelsius™ Technology is an ecosystem of technologies designed to help improve safety and care in the OR. The uniquely intelligent features that define ExcelsiusGPS™, Excelsius3D™, and ExcelsiusHub™ are procedurally integrated to enhance safety and accuracy.

Power Portfolio
The Globus Medical Power Portfolio is engineered to seamlessly integrate with enabling technology to streamline surgical workflow and address clinical challenges. The portfolio drives efficiency at every level and helps to safeguard critical and delicate anatomy through navigation for enhanced visualization and through innovative technologies like the DuraPro™ Oscillating System, designed to remove bone without wrapping tissue.
Regenerative Biologics
Comprehensive Regenerative Biologic Solutions*
Globus Medical is focused on pioneering innovative regenerative solutions for patients with musculoskeletal disorders. Our comprehensive biologics portfolio includes cellular allografts, synthetic bone void fillers, demineralized bone products, amniotic barriers, graft delivery systems, and autologous biologic centrifuge technologies.
*Indications/applications for use vary by product. Refer to the IFU for comprehensive product information.
Prioritizing Clinical Education
Over the past 20 years, we have evolved our programs in partnership with our renowned faculty. We provide learning experiences for spine surgeons and ALIF access surgeons interested in learning ALIF approaches. Our learning experiences span online educational resources to surgeon-led didactic courses with hands-on training, mentorship opportunities, and field-based training support.
- Drazin D, Kim TT, Johnson JP. Simultaneous lateral interbody fusion and posterior percutaneous instrumentation: early experience and technical considerations. Biomed Res Int. 2015;2015:458284.
- Olsen MA, Mayfield J, Lauryssen C, et al. Risk factors for surgical site infection in spinal surgery. J Neurosurg. 2003;98(2):149-155.
- Olsen MA, Nepple JJ, Riew KD, et al. Risk factors for surgical site infection following orthopaedic spinal operations. J Bone Joint Surg Am. 2008;90(1):62-69.
- Macario A. What does one minute of operating room time cost? J Clin Anesth. 2010;22(4):233-236.
- Shippert RD. A study of time-dependent operating room fees and how to save $100,000 by using time-saving products. Am J Cosmet Surg. 2005;22(1):25-34.
- Evans NT, Torstrick FB, Lee CS, et al. High-strength, surface-porous polyether-ether-ketone for load-bearing orthopedic implants. Acta Biomater. 2015;13:159–167.
- Torstrick FB, Lin ASP, Potter D, et al. Porous PEEK improves the bone-implant interface compared to plasma-sprayed titanium coating on PEEK. Biomaterials. 2018;185:106–116.