Regenerative Biologics
Globus Medical is focused on pioneering innovative regenerative solutions for patients with musculoskeletal disorders. Our comprehensive biologics portfolio includes cellular allografts, synthetic bone void fillers, demineralized bone products, amniotic barrier, graft delivery, and autologous biologic centrifuge technologies.
Cellular Allograft
Our cellular allografts contain viable cells including inherent stem cells and osteoprogenitor cells that are essential requirements for bone formation. Cellular products have many of the same characteristics as the gold standard of bone grafting, iliac crest bone graft, without the morbidity of autograft harvesting. The components of cellular allografts are intended to provide the three essential elements of bone formation: osteogenic cells, osteoconductive scaffold, and osteoinductive growth factors.

Osteocel™ Pro
Osteocel™ Pro is a premier cellular allograft bone matrix designed to mimic the biological profile of autograft.

ViaCell™ Viable Cell Allograft
ViaCell™ is a cellular allograft comprised of partially demineralized cortical fibers and cancellous chips and includes inherent stem cells and osteoprogenitor cells.
Synthetic
Our synthetic biologics are optimized utilizing various technologies such as bovine collagen, bioglass, hydroxyapatite, and β-TCP to aid in bone formation while also delivering excellent handling properties.
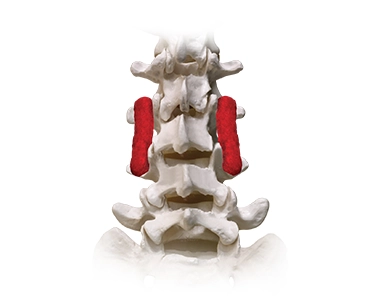
KINEX™ and KINEX™ PLUS Bioactive Bone Void Filler
The components of KINEX™ and KINEX™ PLUS Bioactive are designed to recruit and signal osteoblasts, help promote early vascular development, and bind proteins necessary for bone regeneration. KINEX™ uses The Power of Bioactive Glass™ to help promote bone formation and healing in the posterolateral spine*, pelvis, and extremities.
*KINEX™ Strip, KINEX™ Plus Strip, KINEX™ Putty and KINEX™ Gel are not indicated for use in the spine.
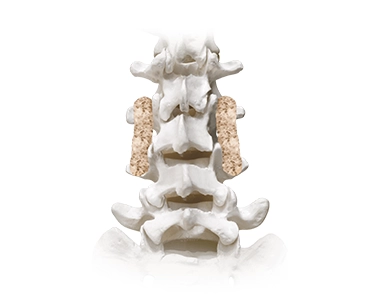
SIGNIFY™ Bioactive Bone Void Filler*
The components of SIGNIFY™ Bioactive are designed to recruit and signal osteoblasts that help promote bone formation. SIGNIFY™ provides an osteoconductive matrix to allow for new bone growth and contains activating chemistry designed to attract and stimulate bone cells.
*SIGNIFY™ Putty and Gel are not indicated for use in the spine.
Attrax™ Putty
Attrax™ Putty is a synthetic bone void filler designed to drive bone formation in the posterolateral spine, intervertebral disc space, and pelvis with a uniquely engineered bioactive and osteoconductive surface structure.
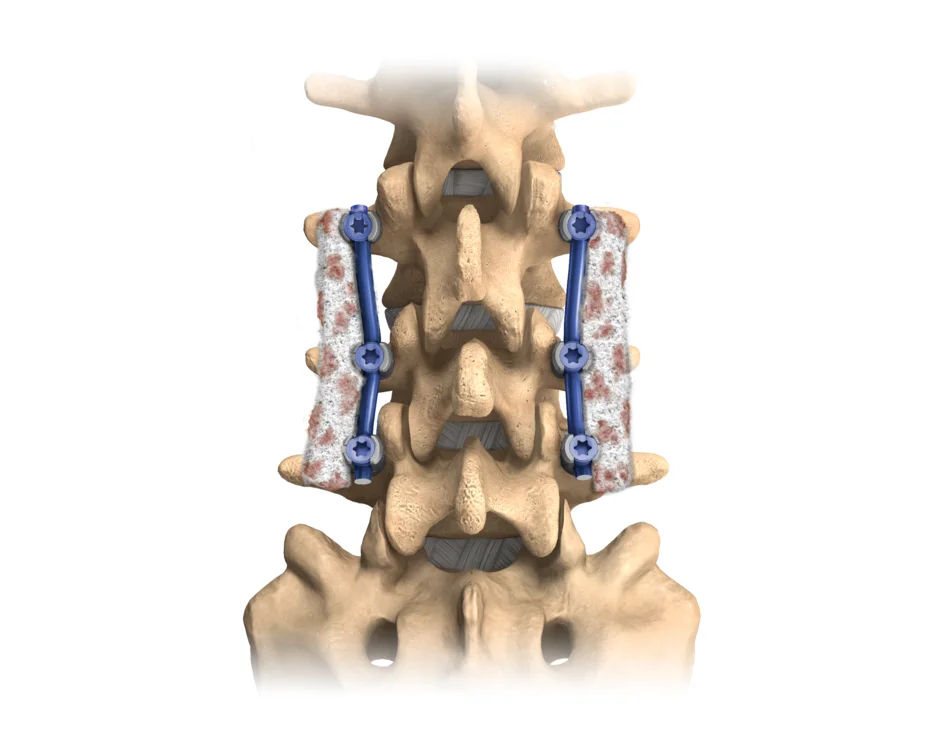
Attrax™ Scaffold
Attrax™ Scaffold is a synthetic, osteoconductive, resorbable bone void filler for the repair of bony defects in the posterolateral spine, intervertebral disc space, or pelvis, consisting of tricalcium phosphate/hydroxyapatite granules pre-mixed with type I bovine collagen.
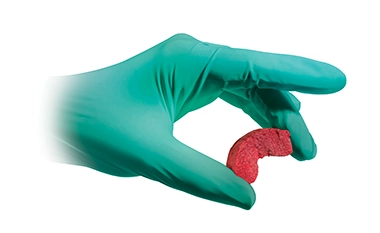
Formagraft™ Collagen Bone
Graft Matrix
Formagraft™ Collagen Bone Graft Matrix is designed to provide a highly absorbent, compression-resistant matrix. Comprised of purified type 1 collagen and biphasic (β-TCP/HAp) granules, Formagraft™ delivers excellent handling properties and a controlled degradation rate.1
DBM/Allograft
Our DBM/allograft biomaterials provide a natural osteoconductive scaffold composed of 100% allograft bone without any added synthetic carrier. Use of cancellous bone or bone fibers helps provide a unique architecture with high surface area for osteoconductivity.

ViaSorb™ Demineralized Cancellous Sponges
ViaSorb™ Cubes and Strips are demineralized cancellous sponges that provide a natural osteoconductive scaffold with unique compressive capabilities that facilitate packing into bony voids and within allograft spacers.
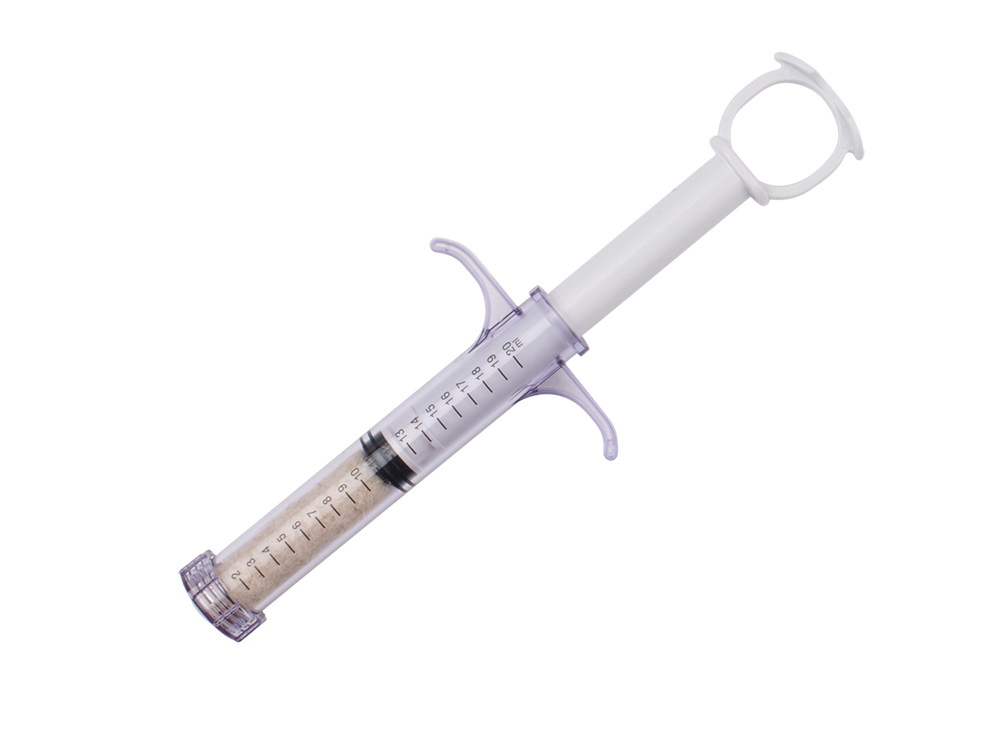
Ossifuse™ HSA Fiber Bone Graft
Ossifuse™ HSA Boats, Strips, and Putty are composed of 100% allograft bone without an added synthetic carrier. The demineralized cortical fibers in Ossifuse™ HSA have a unique architecture with a high surface area for cellular attachment and growth factor release.

Cancellous Chips
Available as cancellous chips, crushed cancellous chips, cortical cancellous chips, and demineralized cancellous chips, traditional bone allograft is derived from donated human cadaveric tissue and serves as a scaffold for new bone formation.
Amniotic Membrane
Our dual layer amnion patch is processed from human placental tissue. The protective properties of amnion make it an ideal barrier to protect soft tissue from the surrounding environment.

ViaShield™ Dual Layer Amnion Patch
ViaShield™ is a chorion-free, dual layer amnion patch processed from human placental tissue. The placental tissue is derived from live healthy donors at childbirth. The protective properties of amnion make it an ideal barrier to protect soft tissue from the surrounding environment.
Graft Delivery
Our graft delivery systems are designed for targeted delivery of graft materials in MIS applications. These systems allow for controlled delivery to streamline surgical workflow.

InstaFill™ Graft Delivery System
The InstaFill™ Graft Delivery System is designed to load and deliver synthetic bone graft, allograft, and autogenous bone graft quickly and efficiently.
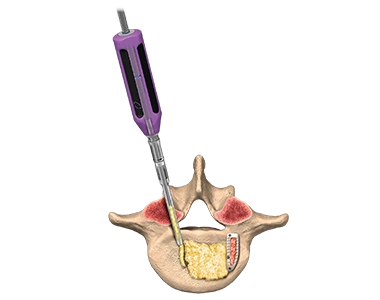
Graft Delivery System™
The Graft Delivery System™ is designed specifically for the delivery of Osteocel™ grafts into the disc space in minimally disruptive MAS™ PLIF/TLIF procedures.
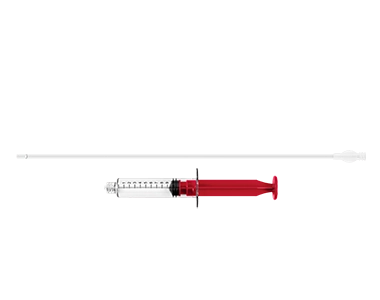
ALLOCATE™ Cannula Kit
The ALLOCATE™ Cannula Kit provides instruments for consistent and controlled delivery of KINEX™ PLUS Bioactive Gel.
Autologous PRP/BMAC™/Adipose
The Harvest™ SmartPrep™ Centrifuge System is a FDA-cleared autologous biologic spin-down device capable of preparing bone marrow aspirate concentrate (BMAC™), platelet-rich plasma (PRP), and adipose-derived cellular biologics (AdiPrep™) in a seamless single-button operation. The flexibility in treatment options offers the ability to choose a biologic solution based on patient needs.
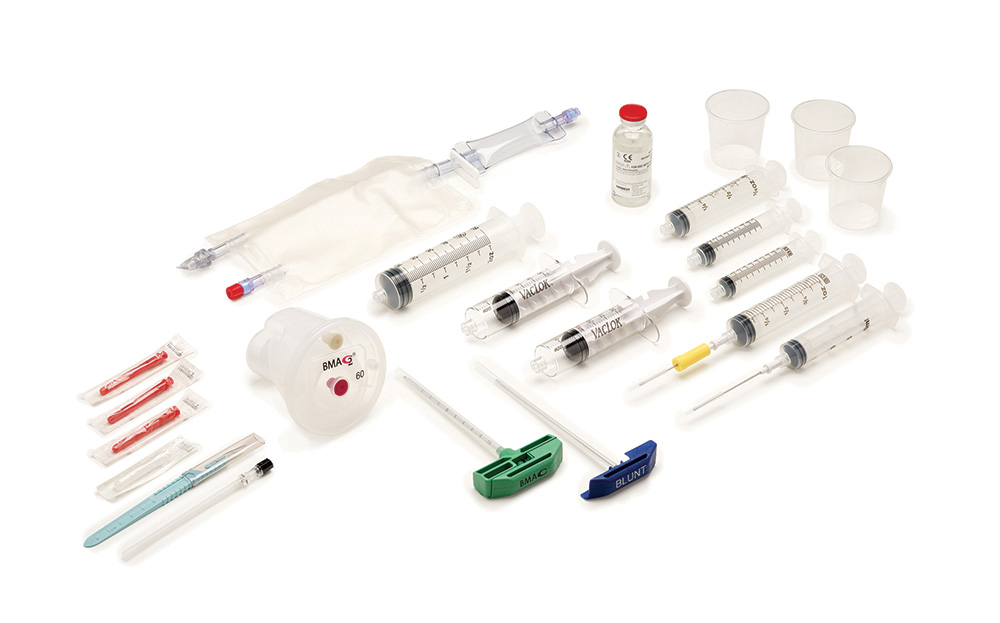
Harvest™ SmartPrep™ 3 System
The Harvest™ SmartPrep™ 3 System is an autologous biologic centrifuge platform that generates highly concentrated platelet-rich plasma (PRP), bone marrow aspirate concentrate (BMAC™), and adipose-derived (AdiPrep™) cellular biologics.
- Walsh R, Vizesi F, Cornwall G, et al. Posterolateral spinal fusion in a rabbit model using a collagen-mineral composite bone graft substitute. Eur Spine J. 2009;18(11):1610–1620.
