Cohere™
Porous PEEK
Manufactured through a proprietary extrusion process, the first-of-its-kind porous architecture is designed to promote bone ingrowth1 while maintaining the biomechanical1 and imaging properties of PEEK. Adhering to the three core principles of Advanced Materials Science™—surface, structure, and imaging—Cohere™ combines the inherent benefits of porosity with the advantageous material properties of PEEK to create implants intelligently designed for fusion.
Watch Now
Patients
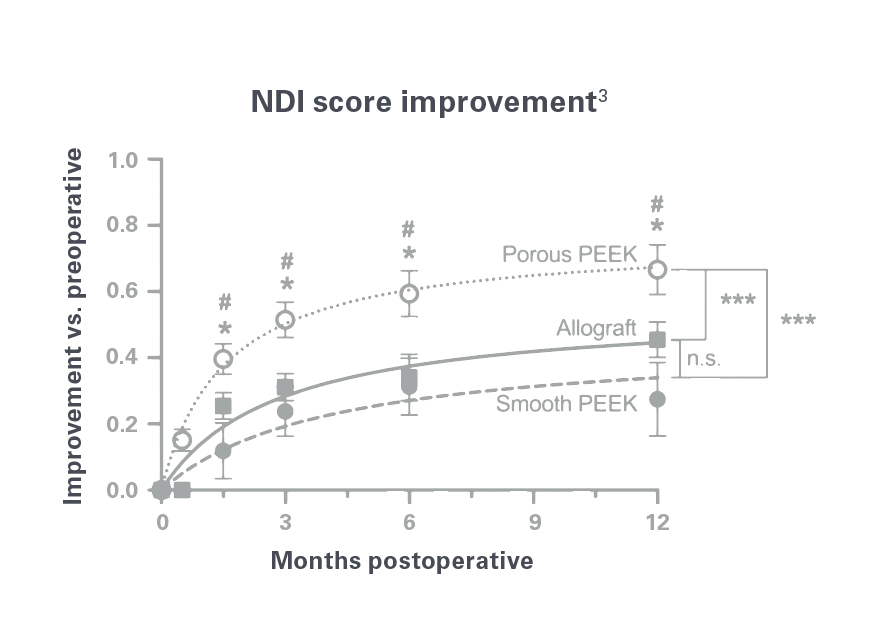
Cohere™ Cervical interbody spacers have shown significantly better NDI and neck pain outcomes than structural allograft or smooth PEEK implants as early as 6 weeks.3

Surgeons
Cohere™ implants have demonstrated >3x the integration strength of smooth, solid PEEK implants as demonstrated in animal models.2

Hospitals
Cohere™ avoids the need for in-hospital sterilization through sterile packaged implants.
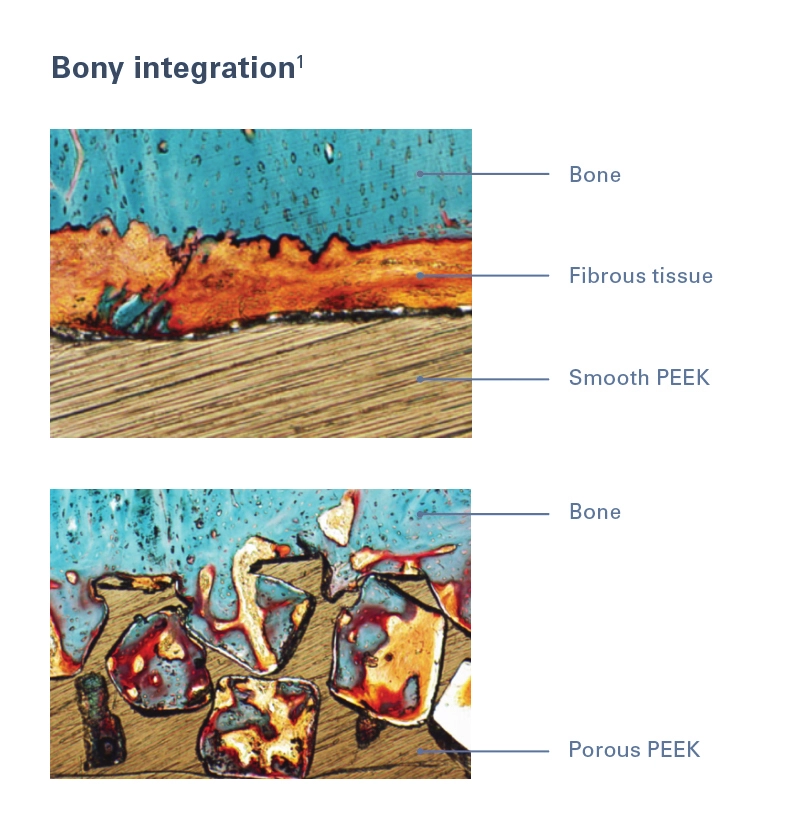
Porosity Drives Ingrowth
The proprietary porous surface technology featured in porous PEEK implants has been shown to elicit a biological response in preclinical animal models,1 reducing fibrous tissue growth observed in smooth PEEK implants.1
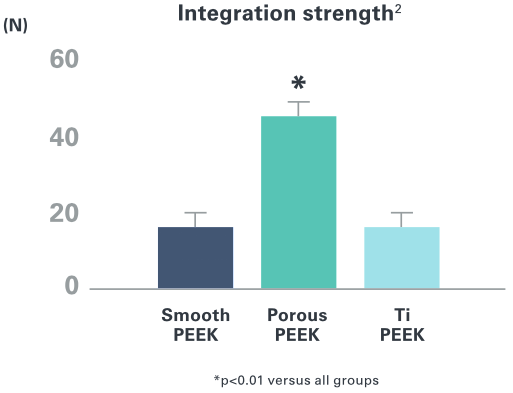
Porosity Drives Integration Strength
Interconnected porous PEEK scaffold supports strong mechanical interlock through bone ingrowth in preclinical animal models. 1-2
Porosity Drives Outcomes
Significant improvements in NDI and neck pain have been demonstrated with porous PEEK vs. smooth PEEK and structural allograft in anterior cervical discectomy and fusion as early as 6 weeks.3
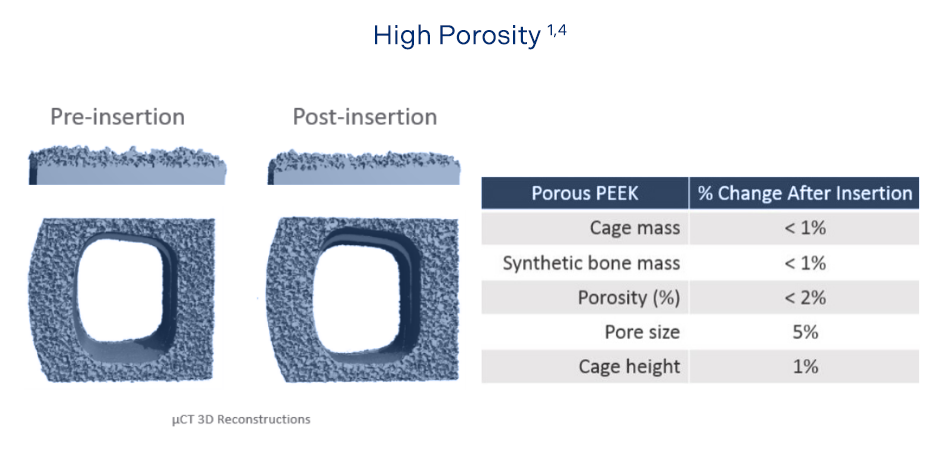
Durable Porous Structure
Cohere™ porous technology maintains high porosity under conditions that replicate anatomic loading and resists abrasion damage and delamination under impacted insertion.1,4
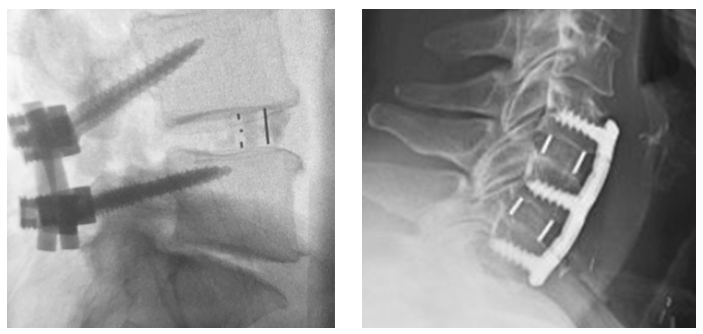
Clear Imaging for Postoperative Confirmation of Fusion
Created with imaging in mind, Cohere™ leverages the radiolucent properties of PEEK to enable radiographic visualization on a variety of imaging modalities. This provides surgeons with the ability to assess placement of the implant intraoperatively and confirm fusion postoperatively.
For important product safety information on each of the implants in the porous PEEK portfolio, including, but not limited to, indications for use, contraindications, warnings, precautions, and potential adverse effects, please see the product-specific Instructions for Use, which can be found at nuvasive.com/eifu.
- Evans NT, Torstrick FB, Lee CS, et al. High-strength, surface-porous polyether-ether-ketone for load-bearing orthopedic implants. Acta Biomater. 2015;13:159–167.
- Torstrick FB, Lin ASP, Potter D, et al. Porous PEEK improves the bone-implant interface compared to plasma-sprayed titanium coating on PEEK. Biomaterials. 2018;185:106–116.
- Hill CP and Strenge KB. Early clinical outcomes comparing porous PEEK, smooth PEEK, and structural allograft interbody devices for anterior cervical discectomy and fusion. J Spine Neurosurg. 2019;8(1):1–7.
- Torstrick FB, Klosterhoff BS, Westerlund LE, et al. Impaction durability of porous polyether-ether-ketone (PEEK) and titanium-coated PEEK interbody devices. Spine J. 2018;18:857-865.