Advanced Materials Science™
Porous Titanium | Porous PEEK
Altering the surface of implant materials with porosity has been shown to improve fusion.1-5 Through Advanced Materials Science™ (AMS), Globus Medical develops surface and structural technologies designed to enhance the osseointegration and biomechanical properties of implant materials.6-7
Changing Architecture to Enhance Patient Care.
The breakthrough technologies designed through AMS are rooted in three key principles:
Surface
Developing technologies to enhance osseointegration while increasing stability and resistance to expulsion
Structure
Engineering material structures to mimic the elastic modulus of bone while maintaining durability
Imaging
Designing technologies to improve visualization on a variety of imaging modalities
Porous Titanium
Modulus™
Modulus™ integrates endplate porosity with an optimized body lattice structure, providing a fully porous architecture and favorable environment for bone ingrowth.5 Modulus™ enhances visualization compared to traditional titanium interbody implants in a variety of imaging modalities. Modulus™ is available in a range of sizes and lordotic options for ALIF, XLIF™, TLIF, and cervical.

HEDRON™
HEDRON™ 3D-printed titanium interbody spacers feature a biomimetic porous scaffold designed to promote bone formation onto and through the implant. In a preclinical ovine study, the porous architecture of HEDRON™ showed significantly more bone growth compared to PEEK and titanium implants at 6 weeks postoperative.8
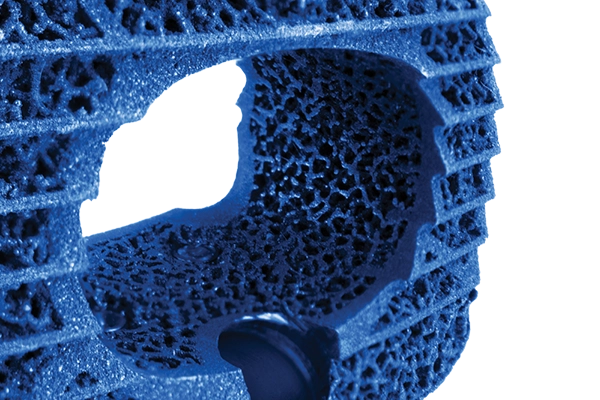
Porous PEEK
Cohere™
Proprietary Cohere™ technology introduces a first-of-its kind porous architecture designed to promote bone ingrowth7 while maintaining the biomechanical and imaging properties of PEEK. Cohere™ is available in a dynamic range of heights and lordotic options for TLIF, XLIF™, and cervical.

- Smith WD. Early outcomes of titanium surface textured, 3-dimensional manufactured implants for lumbar spinal fusion. Presented at: SOLAS 11th Annual Meeting; May 17-19, 2018; San Diego, CA, USA.
- Hill CP and Strenge KB. Early clinical outcomes comparing porous PEEK, smooth PEEK, and structural allograft interbody devices for anterior cervical discectomy and fusion. J Spine Neurosurg. 2019;8(1):1-7.
- Burkus JK. Early outcomes of anterior cervical discectomy and fusion using a porous PEEK interbody fusion device. J Spine Neurosurg. 2018;7:2.
- Burkus JK, Rehak C. Anterior cervical discectomy and fusion using porous PEEK implants at levels adjacent to a previous fusion. J Spine Neurosurg. 2019;8:3.
- Preclinical data on file. Data may not be representative of clinical results. TR 9604787.
- Preclinical data on file. Data may not be representative of clinical results. TR 9604781.
- Torstrick FB, Lin ASP, Safranski DL, et al. Effects of surface topography and chemistry on polyether-ether-ketone (PEEK) and titanium osseointegration. Spine. 2020;45(8):E417-E424.
- Animal study data on file.
For important product safety information, please visit globusmedical.com/eIFU
