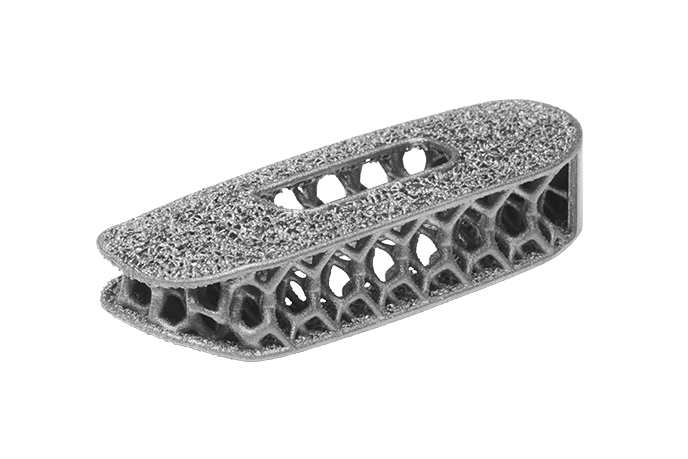
Modulus™
Porous Titanium
Modulus™ is designed through a proprietary optimization algorithm that balances strength and radiolucency. Adhering to the three core principles of Advanced Materials Science™—surface, structure, and imaging—Modulus™ combines the inherent benefits of porosity with the advantageous material properties of titanium to create implants intelligently designed for fusion.
Porous Surface Designed to Participate in Fusion
Porosity elicits a significantly stronger osteogenic response at the cellular level compared to roughened or nano-roughened surfaces1-5 on their own.
A fully porous endplate design promotes new bone ongrowth and ingrowth,1 demonstrating the greatest integration strength by 12 weeks compared to alternative implant materials in an ovine model.1 Microporous endplate architecture maximizes bone-to-implant contact, increasing expulsion resistance.6
The increased surface area and wicking capability of the porous surface is designed to improve blood-to-implant contact compared with traditional interbody implants.
Optimized Lattice Structure
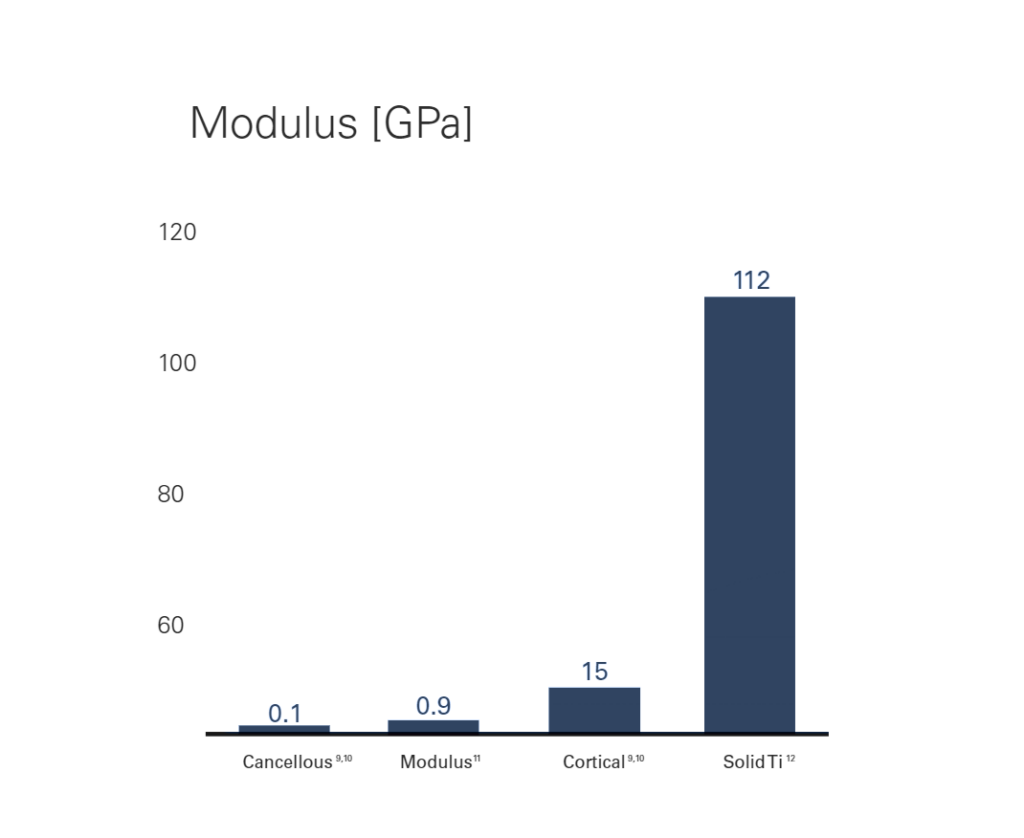
Modulus™ implants are designed with an optimized balance between strength and stiffness to minimize stress shielding7 and subsidence.8 The implants implement a proprietary design optimization methodology, generating a unique internal architecture for a desired effective modulus that is similar to bone.
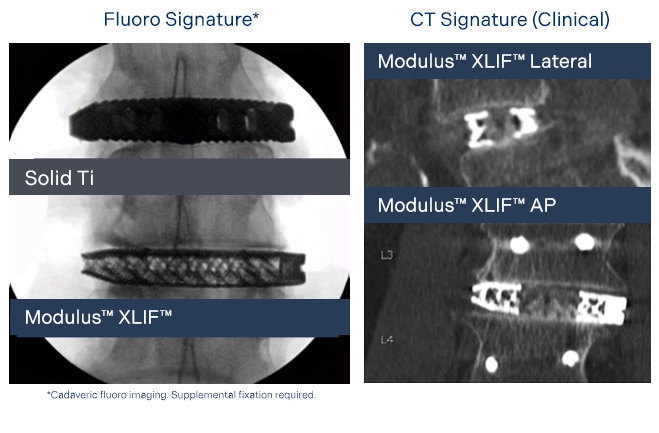
Enhanced Imaging Characteristics for Visualization of Fusion
Created with imaging clarity in mind, the proprietary implant design and manufacturing process allow enhanced visualization on a variety of imaging modalities.
For important product safety information on each of the implants in the Modulus™ portfolio, including, but not limited to, indications for use, contraindications, warnings, precautions, and potential adverse effects, please see the product-specific Instructions for Use, which can be found at nuvasive.com/eifu.
- Preclinical data on file; data may not be representative of clinical results. TR 9604787.
- Cheng A, Humayun A, Cohen DJ, et al. Additively manufactured 3d porous ti-6al-4v constructs mimic trabecular bone structure and regulate osteoblast proliferation, differentiation and local factor production in a porosity and surface roughness dependent manner. Biofabrication. 2014;6:045007.
- Cheng A, Humayun A, Boyan BD, et al. Enhanced osteoblast response to porosity and resolution of additively manufactured ti-6al-4v constructs with trabeculae-inspired porosity. 3D Print Addit Manuf. 2016;3:10–21.
- Cheng A, Cohen DJ, Kahn A, et al. Laser sintered porous ti-6al-4v implants stimulate vertical bone growth. Ann Biomed Eng. 2017;45:2025–2035.
- Cheng A, Cohen DJ, Boyan BD, et al. Laser-sintered constructs with bio-inspired porosity and surface micro/nano-roughness enhance mesenchymal stem cell differentiation and matrix mineralization in vitro. Calcif Tissue Int. 2016;99:625–637.
- Preclinical data on file; data may not be representative of clinical results. TR 9603905.
- Chatham LS, Patel VV, Yakacki CM, et al. Interbody spacer material properties and design conformity for reducing subsidence during lumbar interbody fusion. J Biomech Eng. 2017;139(5):051005.
- Preclinical data on file; data may not be representative of clinical results. TR 9603972.
- Hou FJ, Lang SM, Hoshaw SJ, et al. Human vertebral body apparent and hard tissue stiffness. J Biomech. 1998;31(11):1009–1015.
- Ladd AJ, Kinney JH, Haupt DL, et al. Finite-element modeling of trabecular bone: comparison with mechanical testing and determination of tissue modulus. J Orthop Res. 1998;16(5):622–628.
- Preclinical data on file; data may not be representative of clinical results. TR 9604781.
- Elias CN, Lima JH, Valiev R, et al. Biomedical applications of titanium and its alloys. JOM. 2008;60(3):46–49.