ACDF
Anterior Cervical Discectomy and Fusion
Through the combination of our extensive interbody portfolio, competitive plate offerings, retractor systems, and biologics portfolio, we offer best-in-class anterior cervical discectomy and fusion (ACDF) procedural solutions.
Reimagining Cervical, Starting with ACDF
The Globus ACDF portfolio offers optimized visualization through our access systems, a complete interbody portfolio featuring Advanced Materials Science™ and Expandable Technology™, and versatile fixation options to accommodate varying patient anatomy and surgeon preference.
Portfolio Highlight
The Anterior Cervical Plate (ACP) system is an ultra-thin plate system designed to help achieve ACDF surgical goals through an optimized balance of profile and stiffness for each surgical level.
Access
Optimized Exposure for ACDFs
Proper exposure in an ACDF is one of the keys to good clinical outcomes. Our anterior cervical retractor systems are designed for workflow reproducibility, patient safety, and surgeon confidence.
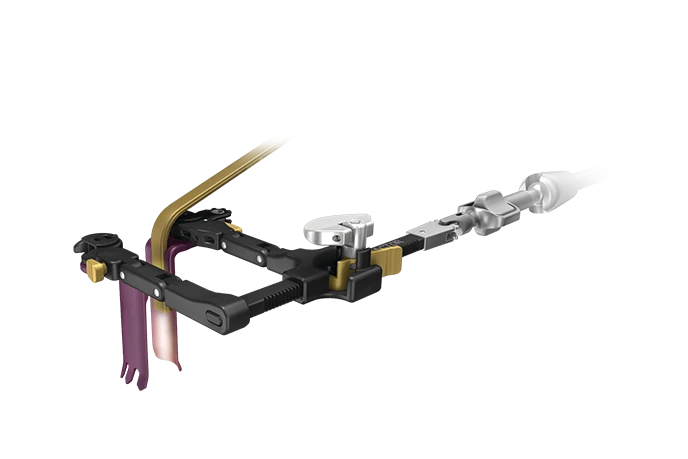
MaXcess-C™
The MaXcess-C™ retractor body is designed to be low profile, accommodating for larger instrumentation and surgeon working angles. The connection mechanisms are designed to simply and confidently engage the blades to the retractor body. Friction joints are engineered for a consistent level of resistance to joint movement so as not to require constant upkeep and adjustments.
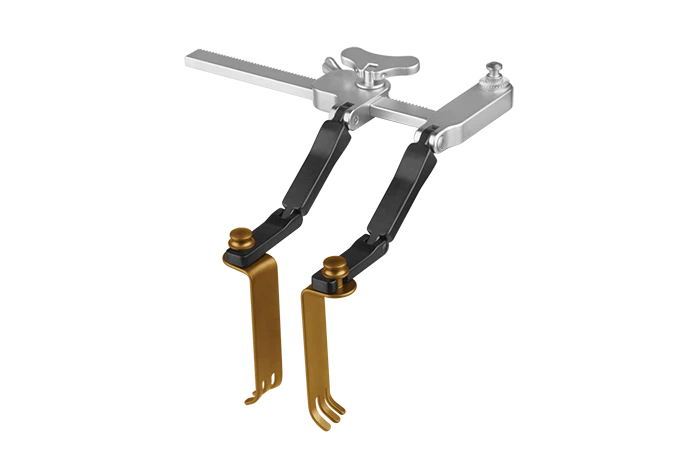
MARS™ ACDF
The MARS™ ACDF Cervical Retractor has a small, sleek, stainless-steel body with side-loading radiolucent titanium blades. A wide range of blade offerings are available to adapt to patient anatomy for intraoperative versatility.
Interbody Implants
Outcome-Driven Static Interbody Spacers
Our static interbody spacer portfolio includes Advanced Materials Science, titanium plasma sprayed (TPS), and traditional spacer offerings. Highlights include the Advanced Materials Science portfolio with technologies that are designed to enhance the osseointegration and biomechanical properties of PEEK and titanium1,2 and our TPS that offers the benefits of PEEK with a titanium bony interface that allows for clear postoperative evaluation.

Cohere™ Cervical
Cohere™ Cervical is a porous PEEK interbody spacer designed to promote bone ingrowth3 while maintaining the biomechanical3 and imaging properties of PEEK.
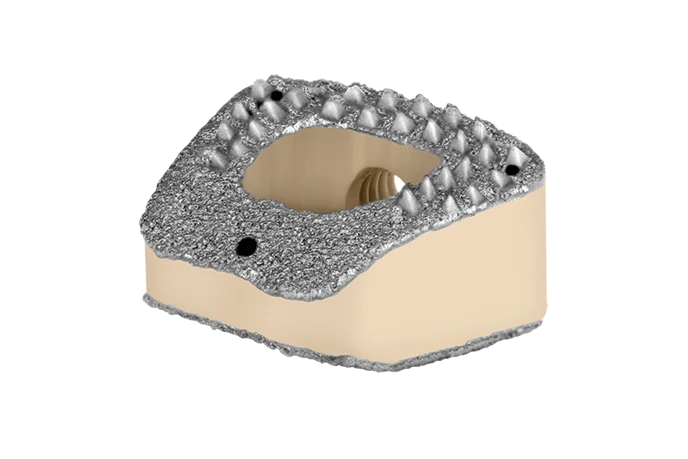
COLONIAL™
The COLONIAL™ spacer is an anterior cervical interbody spacer made from PEEK radiolucent polymer, while COLONIAL™ TPS combines radiolucency with a titanium plasma spray (TPS) coating on the endplate-contacting surfaces. They are available in numerous footprints, heights, and sagittal profiles to accommodate various patient anatomies.
3D-printed Ti offerings include:

HEDRON C™
HEDRON C™ is a 3D-printed anterior cervical interbody fusion spacer that features a biomimetic porous scaffolding designed to promote bone formation onto and through the implant.4

Modulus™ Cervical
Modulus™ Cervical is a fully porous Ti cervical interbody spacer designed to provide a favorable environment for bone ingrowth1 while enhancing visualization compared to traditional Ti interbody implants.
Interfixated Interbody Spacers
Our interfixated solutions include integrated stand-alone* plate-spacer options that provide the biomechanical strength of a traditional ACDF while minimizing disruption to patient anatomy. Minimally invasive options with integrated anchor fixation provide fewer procedural steps through a less invasive corridor.
* Restricted indications for standalone use. Refer to the eIFU page for the FDA cleared indications.

HEDRON C-MIS™
The HEDRON C-MIS™ 3D-Printed Integrated Cervical Spacer System is compatible with COALITION MIS™ instruments, and is designed to ensure easy insertion and clear visualization of the disc space. HEDRON C-MIS™ accommodates both COALITION™ screws and COALITION MIS™ anchors* that are delivered inline with the spacer, eliminating the need for angled instruments, particularly at the upper and lower cervical levels where patient anatomy can be challenging. Utilizing HEDRON™ lattice technology, the HEDRON C-MIS™ system is engineered to help promote bone formation onto and through the implant.
* When used with one or more anchors, or when hyperlordotic (≥20°) devices are used, supplemental fixation is required; refer to the IFU for the comprehensive FDA cleared indications and other important device information. eIFU Webpage

HEDRON IC™
HEDRON IC™ is a 3D-printed ACDF spacer that can be used as an interbody spacer itself or combined with the COALITION AGX™ Plate and screws as a stand-alone plate-spacer. HEDRON IC™ features a biomimetic porous scaffolding designed to promote bone formation onto and through the implant.4
Expandable Interbody Spacers
Our expandable interbody technologies are designed to help preserve anatomy and allow for a customizable fit to each patient.

REVEL™-S
REVEL™-S is an all-titanium, expandable stand-alone* ACDF spacer designed to be delivered at a contracted height to reduce impaction and minimize the need for distraction while providing controlled, continuous expansion to help restore disc height.
* When used with two screws, the REVEL™-S Spacer is a stand-alone interbody fusion device intended for use at one or two levels of the cervical spine. When used with any anchors, the REVEL™-S Spacer is intended for use at one level of the cervical spine with additional supplemental fixation such as posterior cervical screw fixation.
Fixation
Versatile Fixation
Our versatile anterior cervical plates are designed for flexibility and variability to accommodate patient anatomy and surgeon preference.
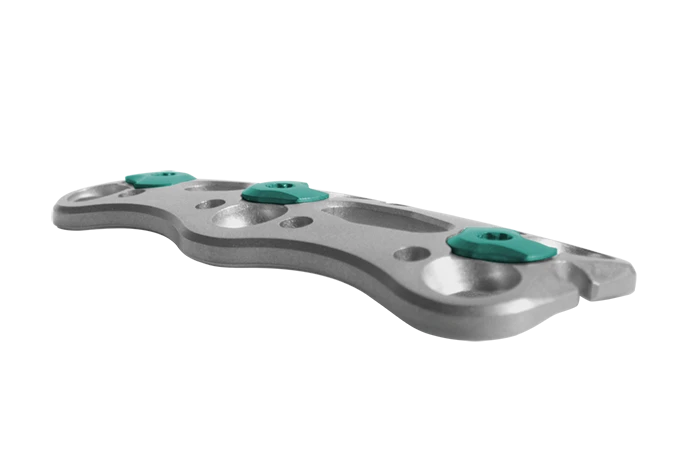
Anterior Cervical Plate (ACP)
The incredibly thin, yet strong ACP system is challenging the notion that current cervical outcomes are good enough. ACP is designed to balance profile and stiffness depending on the levels of every construct.

RESONATE™
RESONATE™ is an extreme angle ACDF plate designed to maximize screw angulation, ease insertion with automatic screw blocking, and provide optimized plate lengths to minimize overlap on the vertebral body.
Regenerative Biologics
Comprehensive Regenerative Biologic Solutions*
Globus Medical is focused on pioneering innovative regenerative solutions for patients with musculoskeletal disorders. Our comprehensive biologics portfolio includes cellular allografts, synthetic bone void fillers, demineralized bone products, amniotic barriers, graft delivery systems, and autologous biologic centrifuge technologies.
*Indications/applications for use vary by product. Refer to the IFU for comprehensive product information.
Prioritizing Clinical Education
Over the past 20 years, we have evolved our programs in partnership with our renowned faculty. We provide learning experiences spanning online educational resources to surgeon-led didactic courses with hands-on training, mentorship opportunities, and field-based training support.
- Preclinical data on file. Data may not be representative of clinical results. TR 9604787.
- Representative coupon, preclinical data on file. Data may not be representative of clinical results. TR 9604781.
- Torstrick FB, Safranski DL, Burkus JK, et al. Getting PEEK to stick to bone: the development of porous PEEK for interbody fusion devices. Tech Orthop. 2017;32:158-166.
- Data on File.