Precice™
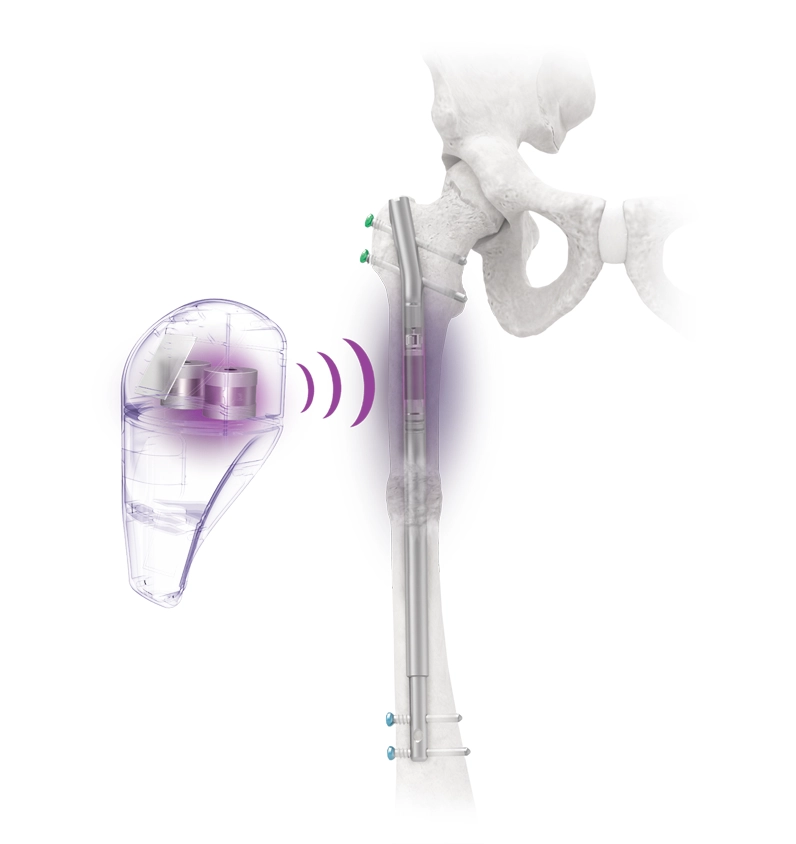
The Precice™ system leverages advanced technology to remotely control an implant from the outside.
Precice™ Applied
The Precice™ system uses distraction osteogenesis. Interaction between magnets in the device and an External Remote Controller (ERC) allows for precise, adjustable, and customizable distraction throughout the lengthening phase of treatment. Following the corticotomy and during the lengthening phase, the Precice™ implant is gradually lengthened based on the patient’s requirements with the handheld ERC.
The patient’s lengthening prescription can be entered into the ERC by the physician. When the desired length is achieved, intramedullary fixation is designed to continue to provide stability throughout the consolidation phase.
Key Benefits
Fewer total procedures
Patients treated with Precice™ for limb length discrepancy (LLD) have fewer surgeries than the previous standard of care.3

Patient preferred
Physical therapy is less challenging, and patients can return to full range of motion (ROM) and daily activity quicker.1,2

Less pain medication
Pain scores and length of time required for prescription pain medication are significantly lower than the previous standard of care.1,2
Avoid operating on the healthy limb
It is no longer required to shorten the healthy limb to avoid pain and issues associated with an external frame.1,2

Quicker healing
It takes an average of 31.3 days to heal with Precice™ compared to 47.1 days with an external device.1,2
Weight bear faster
Patients experience an earlier ability to bear full weight without aids compared to a mono-lateral frame.1,2

Regenerate speed and formation
Regenerate forms faster with less deformity.1,2

Accuracy
Physicians are able to achieve the desired lengthening through ERC technology.1,2
Fewer scars
Patients report feeling significantly happier with fewer scars.1,2
State-of-the-Art Reconstruction Solutions
Precice™ Limb Lengthening System
The Precice™ Intramedullary Limb Lengthening (IMLL) System is an adjustable, state-of-the-art device that utilizes an external, hand-held remote control to non-invasively lengthen the femur or tibia postoperatively. Interaction between magnets in the device and the ERC allows for precise, adjustable, and customizable distraction throughout the lengthening phase of treatment. Following the corticotomy and during the lengthening phase, the Precice™ implant is gradually lengthened externally with the handheld ERC based on patient requirements.
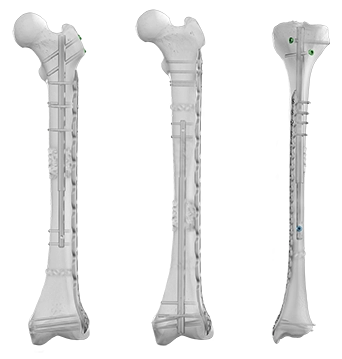
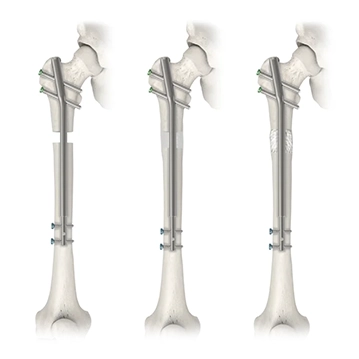
Plate Assisted Bone Segment Transport
Traditional bone transport treatment can be burdensome. Plate Assisted Bone Segment Transport (PABST) is a bone transport technique that combines the Precice™ nail with a locking plate to treat long bone defects, offering a simple, internal solution to treat segmental bone defects caused by a tumor or trauma.
- Laubscher M, Mitchell C, Timms A, et al. Outcomes following femoral lengthening. An initial comparison of the Precice intramedullary lengthening nail and the LRS external fixator monorail system. Bone Joint J. 2016;98-B:1382–1388.
- Landge V, Shabtai L, Gesheff M, et al. Patient satisfaction after limb lengthening with internal and external devices. J Surg Orthop Adv. 2015;24(3):174–179.
- Richardson, S, Schairer W, Fragomen A, et al. Cost comparison of femoral distraction osteogenesis with external lengthening over a nail versus internal magnetic lengthening nail. J Am Acad Orthop Surg. 2019;27(9):e430–e436.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician. Proper surgical procedure is the responsibility of the medical professional. Operative techniques are furnished as an informative guideline. Each surgeon must evaluate the appropriateness of a technique based on his or her personal medical credentials and experience.
The Precice™ Intramedullary Limb Lengthening System is indicated for limb lengthening, open and closed fracture fixation, pseudarthrosis, malunions, nonunions, or bone transport of long bones in patients age 18 years and older and indicated for limb lengthening of the femur and tibia in pediatric patients (greater than 12 years old).
Please refer to the Instructions For Use supplied with the product for specific information on the indications for use, contraindications, warnings, precautions, cautions and sterilization. Instructions For Use are also available by contacting us.