ViaCell® Viable Cell Allograft
Product Overview
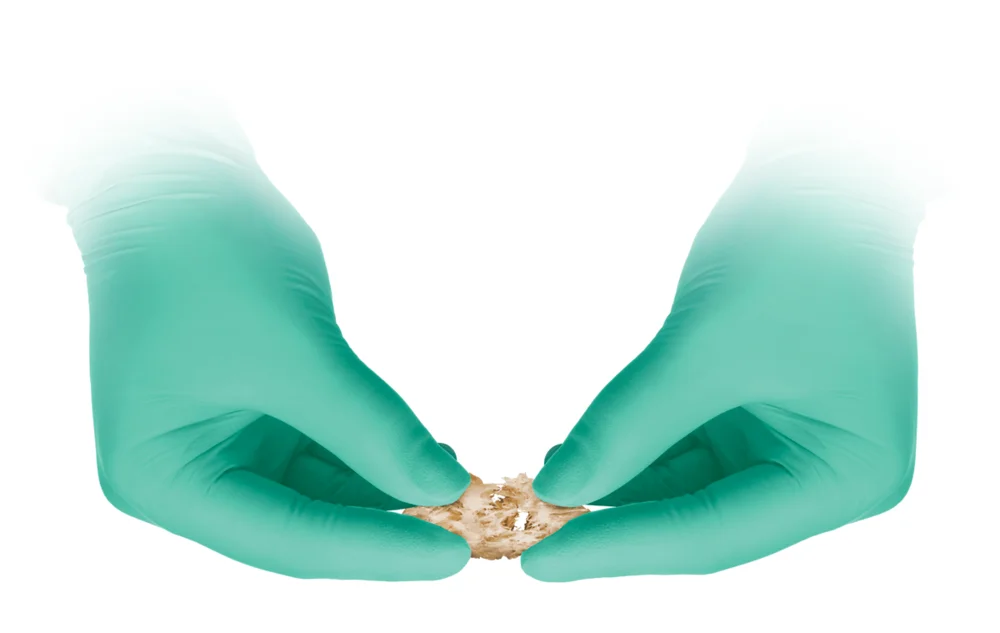
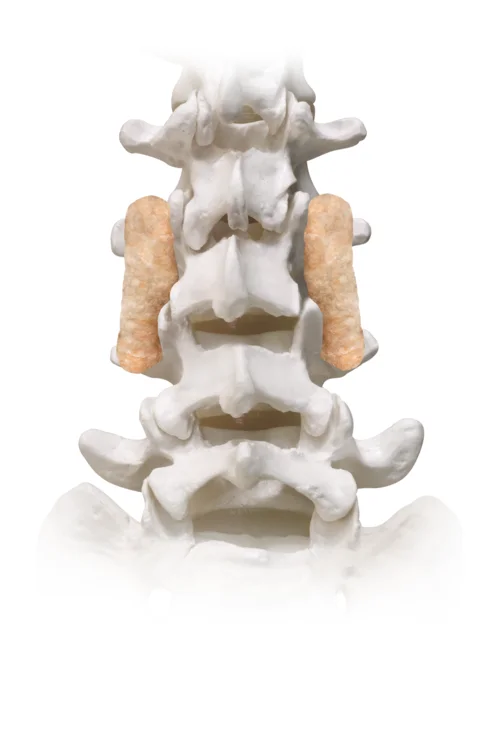
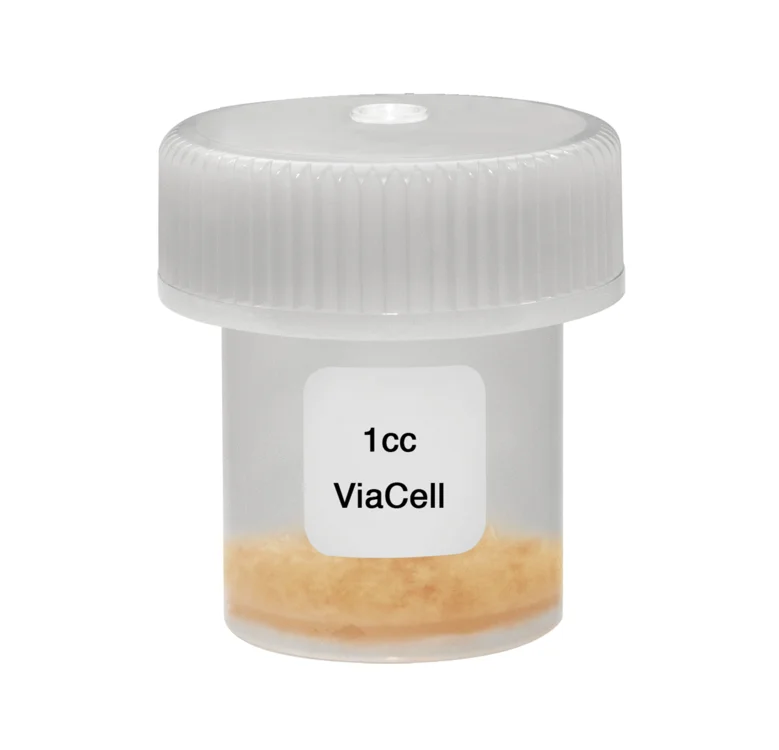
ViaCell® is a cellular allograft containing viable cells including inherent stem cells and osteoprogenitor cells that are essential requirements for bone formation. ViaCell® has many of the same characteristics as the gold standard of bone grafting, iliac crest bone graft, without the morbidity of autograft harvesting. ViaCell® is a cohesive putty that can easily be molded and delivered to the treatment site. The components of ViaCell® provide the three essential elements of bone formation: osteogenic cells, osteoconductive scaffold, and osteoinductive growth factors.
ViaCell® is minimally processed human tissue regulated by the FDA under 21 CFR Part 1271 and Section 361 of the Public Health Service Act.
ViaCell® is processed by Bone Bank Allografts.
Features and Benefits
Viable Cells
Stem cells play a key role in bone regeneration by differentiating into bone-forming osteoblasts. (1-5)
Exceptional Handling
ViaCell® is a cohesive putty that can easily be molded and delivered to the treatment site.
Optimized Donor Screening
Stringent donor screening standards exceeding AATB and FDA requirements, including donor age requirements, help to ensure cell health and quantity.
1 Yusuke S et al. Suspended cells from trabecular bone by collagenase digestion become virtually identical to mesenchymal stem cells obtained from marrow aspirate. Blood. 104: 2728-2735, 2004.
2 Knight M et al. Mesenchymal stem cells in bone regeneration. Adv Wound Care 2(6): 306-316, 2013. Review.
3 Tuli R et al. A simple, high-yield method for obtaining multipotential mesenchymal progenitor cells from trabecular bone. Mol Biotechnol. 23(1): 37-49, 2003.
4 Chamberlain G et al. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 25(11): 2739-2749, 2007.
5 Kraus K et al. Mesenchymal stem cells and bone regeneration. Vet Surg. 35(3): 232-242, 2006.