Uniting to advance patient care
Globus Medical and NuVasive have officially joined forces. We’re committed to providing innovative technologies and industry-leading clinical support to help surgeons and healthcare providers deliver better care around the globe. We now have one of the most comprehensive offerings of musculoskeletal procedural solutions and enabling technologies to impact the care continuum. Our employees are relentlessly focused on changing patient lives.
LIFE MOVES US
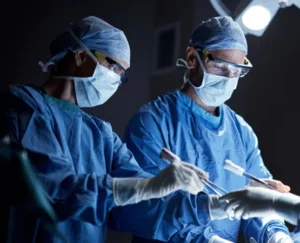
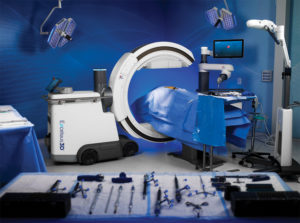
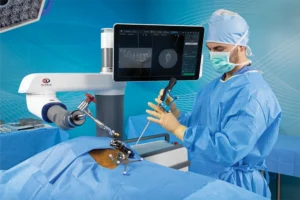
Globus Medical is constantly pursuing improvement in the lives of patients with musculoskeletal disorders with the understanding that speed is critical because life cannot wait.
Why Globus?
At Globus Medical, we move with a sense of urgency to deliver innovations that improve the quality of life of patients with musculoskeletal disorders. Our team is inspired by the needs of these patients, and the surgeons and health care providers who treat them.
We embrace a culture of exceptional response by partnering with researchers and educators to transform clinical insights into tangible solutions. Our solutions improve the techniques and outcomes of surgery so patients can resume their lives as quickly as possible.
The energy and enthusiasm each of us brings every day to Globus is palpable. We are constantly in the pursuit of advancing patient care and understand that speed is critical because life cannot wait.
Join Our Team!
We are seeking talented individuals to develop, manufacture, and distribute state-of-the-art musculoskeletal device products. Globus is inspired by the needs of patients and the surgeons and health care providers who treat them. Our team members help to improve surgical practices and patient quality of life with creative solutions every day.
Featured Careers:
- CNC Machinist
- Project Engineer
- Product Manager
Experience what it’s like to be a valued team member on the cutting edge of medical innovation and patient care.
Our employees enjoy excellent benefits, including:
- Competitive Pay
- Comprehensive Health Coverage (medical/dental/vision/prescription)
- 401(k) with Company Match
- Paid Time Off
- On‐Site Fitness Center